
INTRODUCTION:
We see a vivid array of colours and lights around us in day-to-day life, and chemistry, as a matter of fact, has given us an unparalleled insight to get a feel of this brilliant colour show of Nature inside the laboratories, which has satisfied the chemists from ages. But have we thought about the concept of chemical compounds emitting light? Yes, indeed, that was a great question that has led to the foundation of the domain of luminogenic chemistry and now chemists are on a path to see chemical lights. Sounds cool, right!
GETTING TO KNOW THE STAR:
So, what is this chemiluminescence? This term has a very intuitive definition from our understanding, and this is “ the light emitted as a result of a chemical reaction”. But light emitted is not the only form of energy released, but heat energy may also get released, hence making such reactions endothermic. So, in a nutshell, chemical luminogens or chemical LEDs are those unique compounds that emit light undergoing a chemical reaction.

REASON FOR THIS BEHAVIOUR:
Now that we are fully aware of the phenomena, we all might question: Why do these compounds show such behaviour?
The answer is quite simple if we analyse the dynamics of the reaction. Generally, in reactions, the reactant molecules collide with each other resulting in the formation of transition states which have a high energy configuration. When these transition states disintegrate to give the product molecules, energy is released in different forms. A similar thing occurs in the case of chemiluminescence, where electrons get excited to higher energy levels, and their subsequent de-excitation leads to the release of photons which further emit light, and this decay of the high energy state can be through allowed transitions like fluorescence or forbidden transitions like phosphorescence. But it is worth mentioning that these kinds of reactions have low quantum yield and are hence a little bit difficult to achieve in laboratory conditions.

But chemists have achieved satisfying results in attempts to perform such reactions, and the study of these compounds has opened wide scopes for further research.
HOW IS IT DIFFERENT:
Let us now get to another fundamental, rather important aspect: the difference between chemiluminescence and other luminogenic phenomena that occur in Nature. Simply, chemiluminescence occurs from the electronic transitions occurring in various chemical reactions and, under strict definitive principles, may be called a form of photochemistry.
In some instances, a slight variant of chemical luminogens have been found to occur in the biological kingdom and is popularised by the term “Bioluminescence” which is a beautiful phenomenon taking place in some explicit marine creatures to keep themselves up with the surrounding variations that take place now and then, and these phenomena are believed to be taking place as a result of the chemical reactions between enzymes “Luciferase” and luminescent pigment “Luciferin”, and the low quantum yield of these reactions are counter tackled by the presence of cofactors and some particular proteins.
These examples show chemistry works everywhere!
A VERY RECENT EXAMPLE AND ITS CHEMISTRY:
Now comes the crispy and solid part! Let us fasten our seat belts and enjoy a beautiful journey:
A good previously known example of a luminogenic compound is “Luminol”, with excellent luminescent properties.
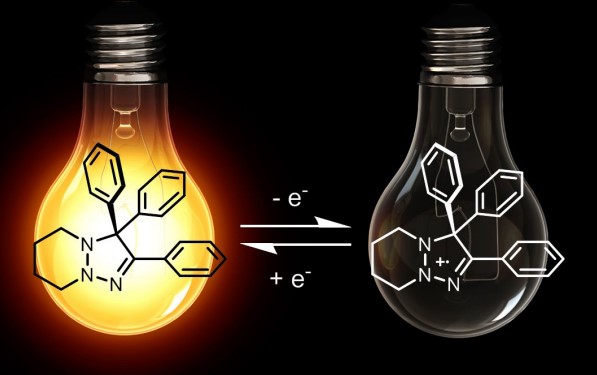
But now, chemists have found out excellent solid-state emitters with electro-fluoro chromic behaviour. One such recently studied example is the substituted -1,2,3-triazoles. These are extraordinary heterocycles that exhibit fluorescence in solid-state and, depending on the type of substituent, can show solid-state aggregation and hence emission of dazzling light. Previously many such heterocycles were reported, which used to show luminogenic properties, but what is so special about these triazoline derivatives?
They are a sort of molecular switches whose emissivity can be switched on and off by varying the reaction conditions. Cool! And this is possible by converting the species into stable radical cations, which are non-emissive. Still, the neutral compound shows emissive characteristics, and hence these molecules are somewhat more special, showcasing both electro-fluoro chromic behaviour and intrinsic redox activity. So, we can switch on and switch off the compound like the LEDs, bulbs!
Basically, the substituent attached to the heterocyclic moieties lead to a propeller kind of geometry that lead to significant () stacking interactions in solid-state, and thus the compounds get self-quenched.

Now lets us come back to the discussion of the triazoles. So, the chemists were trying to work out the synthesis of triazines from organo-magnesium reagents and functionalised organic azides and by mistake, as they unusual substrate selective reaction of Vinyl Magnesium reagent with azides two times with two different substituents in the vinyl Magnesium reagent and surprisingly the presence of the aryl vinyl magnesium reagent led to the formation of aryl-substituted 1,2,3-triazoline derivatives which showed outstanding electrochemical and photophysical properties and this motivated the further research work.
To the surprise of the chemists working on this, they noticed the importance of the presence of aryl vinyl moiety for getting the desired triazoline derivative as other possibilities lead to the formation of triazene derivatives.

The radical cation and structural analysis were confirmed by X-ray crystallography and EPR spectroscopy, and these radical cations were found to be relatively stable, and they were formed by the treatment of the neutral triazoline derivative with a combination of Silver Nitro ditriflate[Ag(NTf2)] and tetrahydrofuran as a solvent.

WHAT DO WE GET FROM HERE:
In a nutshell, the substituted 1,2,3-triazoline derivatives are really interesting compounds showing unique characteristics conversion into non-emissive radical cations under significantly low potentials. The radical cations are quite stable and can be used for different spectroscopic analyses. These compounds can be used for the preparation of novel electrochromic devices. They can also be used in imaging techniques using scanning fluorescent microscopes.
SOME MORE IMPLICATIONS:
So far, we have completed our journey to get to know about some recent star compounds in this domain. Let us now dive into the world of glowing fireflies; sounds pretty aesthetic and romantic, but this glowing and wiggling around in the nights is all due to simple Peroxide decomposition reactions, which involve the transition of singlet oxygen to triplet ones that lead to this bioluminescence phenomena.

Furthermore, many species of luminescent fungi are known now that contain compounds undergoing a cascade of peroxide decompositions in specific carbonyl moieties leading to the glow that is observed.

APPLICATIONS:
We have come a long way, having seen the different aspects of chemiluminescence. It is now time to see some applications of this concept :
- One of them can be as an analytical tool for trace gas measurements.Effective Nitrogen and Sulphur detectors and outstanding candidates for gas chromatography and capillary electrophoresis.
- Excellent compounds for clinical immunoassays, protein-nucleic acid, blotting, monitoring reactive oxygen species, looking at drug interactions inside the body of organisms.
- Can be used as effective molecular switches in the study of reactions and their kinetics and dynamics.
CONCLUSION:
We have come to an end of this beautiful journey, and it is worth mentioning that chemiluminescence is a fascinating and unique domain of research and further studies as many more compounds are still left for the chemists for us to ponder upon and spend some joyful time in understanding their reactivity. The study of the 1,2,3-triazoline derivatives has also taught us that mistakes can often do wonders in experimental science!
REFERENCES:
- “Highly-Substituted 1,2,3-Triqzolines: a New Class of Solid State Emitters with Electrofluorochromic Behaviour”
-WILEY-VCH, Abdusalom A. Suleymanov.
- “THE CHEMILUMINESCENCE OF ORGANIC COMPOUNDS”
- FRANK McCAPRA, School of Molecular Sciences, University of Sussex, Falmer, Brighton.
- WEBSITES CONSULTED:
“Thought Co.”-Anne Marie Helmenstine, PhD.
“Clinical applications of chemiluminescence”-ScienceDirect.com.
About the Author:

I am Subhajit Chakraborty, a 2nd-year BS-MS student at IISER K, INSPIRE fellow, NSEC gold medalist. I am an enthusiastic science geek who loves chemistry and aspires to do something in Natural Product Synthesis, Pericyclic chemistry, and Photochemistry. Apart from studies, I love to sing and entertain my friend circle with lovely songs. This is my first blog on TQR’s platform.