
Prospecting over a panoramic perception, what matters most is the matter itself. It is, therefore, not a surprise that chemistry, the science of matter, is considered by many as the central science residing between physics and biology. Its power derives from its ability to analyze and synthesize molecules from atoms and other, more or less complex, molecules. Organic synthesis is utterly the art and science of replicating the molecules of living nature and creating others like them in the laboratory. Synthetic organic chemists have the vigour to replicate some of the most intriguing molecules of living nature in the laboratory and apply their developed synthetic strategies and technologies to construct variations of them.
Let’s kingpin the concept of ‘retrosynthesis’
Proficient synthetic organic chemists have sanctioned it as the ‘heart of organic synthesis’. The systematic approach for designing a synthetic route to a molecule is to subject a target molecule to an intellectual exercise called ‘retrosynthetic analysis’. This algorithm aims for structural simplification. It is well suited for discovering different synthetic routes and comparing them in a logical and candid fashion. A target molecule is smoothly deconstructed into readily available starting materials by means of disconnection and functional group interconversion (FGI). Heterocyclics have been readily scrutinized in this field. However, a chemist has to be well conscious of stereoselectivity as well as chemoselectivity. The usual strategy employed to allow for such selective differentiation of the similar groups is to convert each group to a masked (protected) form that is not reactive but can be unmasked (deprotected) to yield the group when necessary.
The latter part of the 20th century witnessed impressive advances in the area of new synthetic methodology, which propelled the art of organic synthesis to higher levels of elegance, practicality and efficiency. These new methods facilitated discovery research, product development and manufacturing of pharmaceuticals and other fine chemicals that benefited society.
Among the most powerful of these useful reactions are the Wittig reaction for constructing carbon-carbon double bonds, developed by German chemist Georg Wittig, and the hydroboration reaction, developed by American chemist Herbert C. Brown. Brown and Wittig shared the 1979 Nobel Prize in Chemistry ‘for their development of the use of boron and phosphorus-containing compounds, respectively, into important reagents in organic synthesis.’
Needless to mention, the contributions of English chemist Sir Derek H. R. Barton (FRS) and Norwegian chemist Odd Hassel to conformational analysis played a major role in shaping our understanding of molecular structure that facilitated chemical reactivity and selectivity. Barton’s discoveries extended well beyond stereochemistry and into other domains of organic synthesis such as biomimetic oxidative coupling reactions and radical chemistry.
However, the quantum leap materialized when the Stork-Eschenmoser hypothesis set the floor on fire. Eschenmoser’s contributions to heterocyclic synthesis are equally impressive and include regio and enantioselective reactions, method development, corrin chemistry and the aforementioned landmark total synthesis of vitamin B12. The pride moment for India stands tall when phosphate and amide bond-forming processes were discovered by Indian-born American biochemist H. Gobind Khorana, leading to diverse pathways of synthetic approaches.
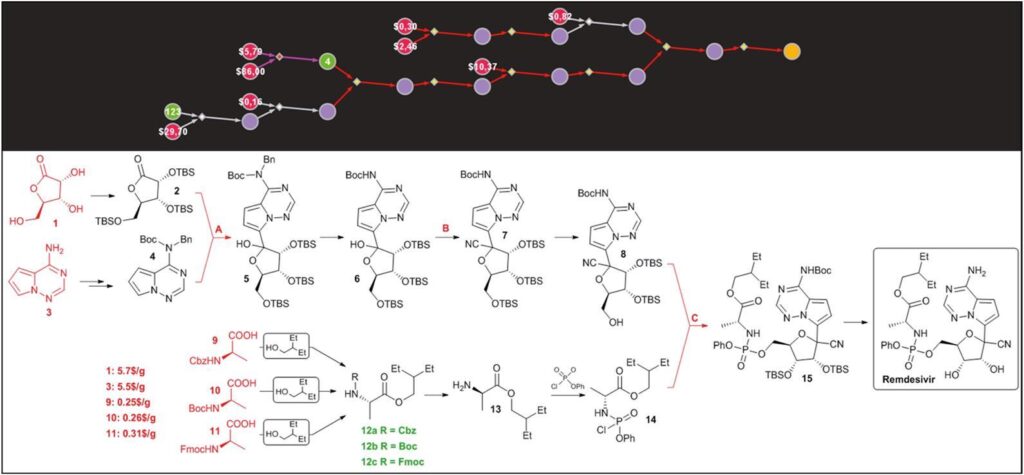
But you know what, the contributions set up don’t culminate over this point. Chematica, a retrosynthetic software tool driven by artificial intelligence, has been used to develop synthetic contingency plans to produce drugs on a large scale in case of market shortages. The program devised multiple routes to hydroxychloroquine and remdesivir, the latter of which has shown promise in treating patients with Covid-19. Researchers are encouraged in industry and academia to use programs like Chematica to develop contingency plans for other drugs.
“If there is a tool that can be used to give you ideas, and it only takes 15-20 minutes, then why not?”

Calicheamicin 
Taxol
Say “Hi” to Calicheamicin, a fascinating molecule whose intrigue stems from not only its phenomenal cytotoxic properties and potential as an anti-cancer agent but also its stunning molecular architecture and fascinating mechanism of action. This mechanism can be compared to that of a guided missile in which the enediyne moiety acts as the explosive payload (generating reactive benzenoid diradicals through Bergman cycloaromatization). In fact, chemists had no idea at the outset whether they could ever arrive at their destination, for the unpredictable and formidable challenges, owing to the demonic complexity of the molecule and its potential chemical instability.
On the other hand, the legendary cancer curative properties of Taxol are matched by its discovery and development as an anti-cancer drug in the latter part of the twentieth century. In the latter coupling reaction, the β-lactam served as a surrogate to the side chain of Taxol, as expected from the well-known chemistry of this structural motif. The overall synthesis scenario remains circumscribed by stereoselective cycloadditions.
Organic synthesis is the way forward
Ever since its inception, organic synthesis has been on the march, advancing to new levels of performance and reach in terms of molecular complexity and diversity. Its applications have been equally impressive and continue to expand into new domains.
From dyes and pharmaceuticals, organic synthesis came to provide the foundation of a whole new generation of scientific endeavours and industries, including polymers and plastics. Endeavours in total synthesis provided numerous, more or less, complex biologically active molecules for biological and pharmaceutical studies. Thus, from the tiny molecule of urea containing a single carbon atom and no stereogenic site, synthetic organic chemists of our time dare to attempt the synthesis of giant molecules such as that of maitotoxin, the largest secondary metabolite discovered thus far from nature, boasting 164 carbon atoms and 99 sites of stereoisomerism.
Ideally, the ultimate goal of organic synthesis practitioners should be to strive to elevate their art and science to the standards of nature and beyond in terms of efficiency, practicality and elegance. That’s organic synthesis for you, my dear friend. That’s organic synthesis for you.
Yours truly,
A moronic synthon
References:
1. www.chemistryworld.com/news/algorithm-devises-synthetic-contingency-plans-for-covid-19-drug
2. Nicolaou, K. C. & Rigol, S. (2020). Perspectives from nearly five decades of total synthesis of natural products and their analogues for biology and medicine.
3. RSC Publishing House, Royal Society of Chemistry. https://pubs.rsc.org
Proud of you dear💕