
The Journey Begins:
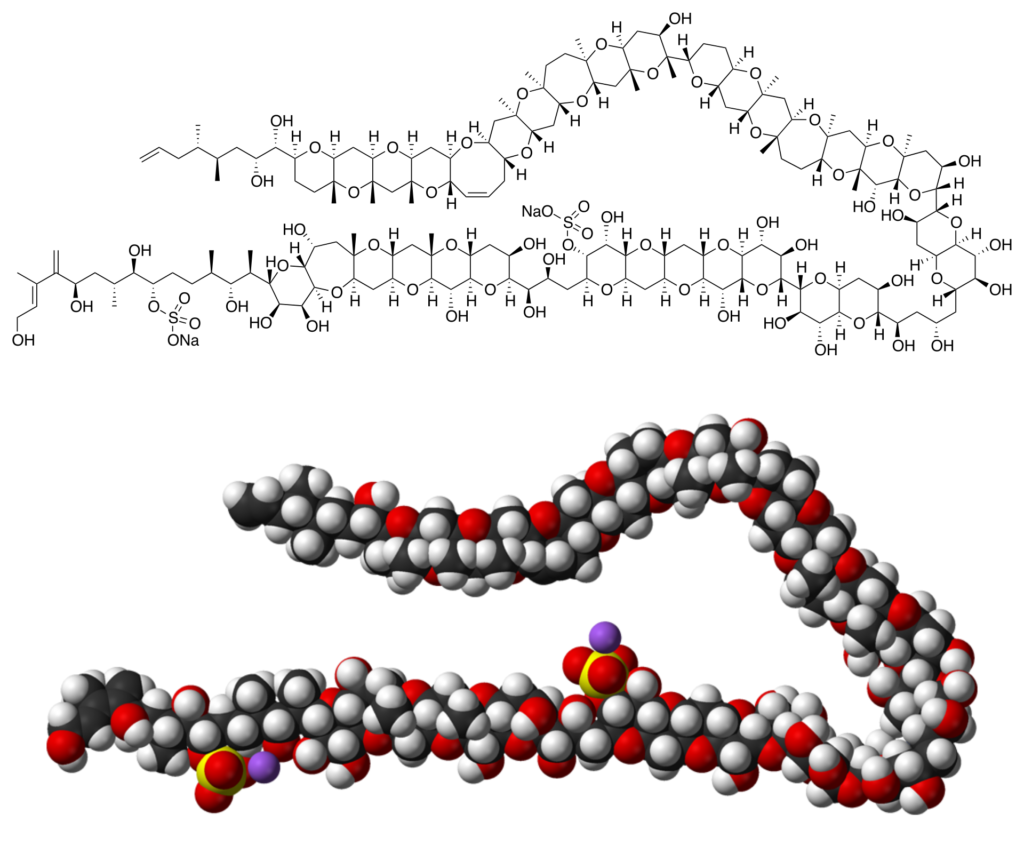
Ever heard of the ‘big papas’ in the world of deadly toxic chemicals? Ever wondered why is it advised not to gulp down a huge amount of coffee, or why chili-pepper causes such an insane burning sensation in our mouth? Well, today’s blog doesn’t feature such simpletons, because get ready and buckle up your seat-belts to shake hands with the largest and the most toxic secondary complex metabolite known to date, ‘Maitotoxin’ aka ‘Agent MTX’ (Chemical formula: C164H256O68S2Na2). This big bro has its elegant molecular architecture, unfurled over 32 heterocyclic rings and 98 freaking stereocentres! The corresponding molecular analogues are water-soluble polyether compounds produced by the dinoflagellate Gambierdiscus toxicus. The breakthrough of urea synthesis by Wöhler inspired chemists to pursue, sharpen, and apply the art of what became known as organic synthesis. No more dramatic has this inspiration been in terms of complexity than in the case of this monarch of marine natural products, Maitotoxin!
Mr. Chemical Biology’s tale: A dip into the deep waters:
Beauty lies in the eye of the beholder, since reality itself is a question of perspective. Recent NMR and computational studies corroborate the initially proposed structure of maitotoxin, involving the stereo-controlled formation of a highly-functionalized eight-membered cyclic ether from an enantiopure camphor-derived bis(spiroepoxide)-1-norbornyl triflate. Rigorous experiments and studies inspired the biological investigation for the ultimate synthetic target and opened the door for discovery and invention in organic synthesis and chemical biology. As part of a program to explore such opportunities, several methods for the construction of maitotoxin’s structural motifs are developed and a number of its polycyclic domains are synthesized.
And if you ask about the potency in toxicity, well we’d say, these toxins can reach upto acute and chronic health effects not just in humans but also in other animal species. Given that these compounds are tasteless, odourless, and heat and acid-stable, conventional food testing methods fail to detect and destroy them in contaminated seafood. Classical ciguateric symptoms include gastrointestinal and neurological disorders, abdominal cramps, diarrhoea, nausea, vomiting, temperature reversal, and itching. These toxins are so deadly that can even be passed on to a foetus or to a newborn child, via placental or breast milk transmission, respectively.
A toxic yet beautiful relationship:
Inspired by its fascinating molecular architecture, synthetic organic chemists began to ponder its synthesis, a goal that seemed at the time far into the future since no existing methods were up to the task. But since this guy possesses the capability to increase the calcium ion influx through excitable membranes, causing cell depolarization, hormone and neurotransmitter secretion, and breakdown of phosphoinositides, playing an impact in regulating the function of integral cell membrane proteins, chemists started to jam along with its unique synthetic art.
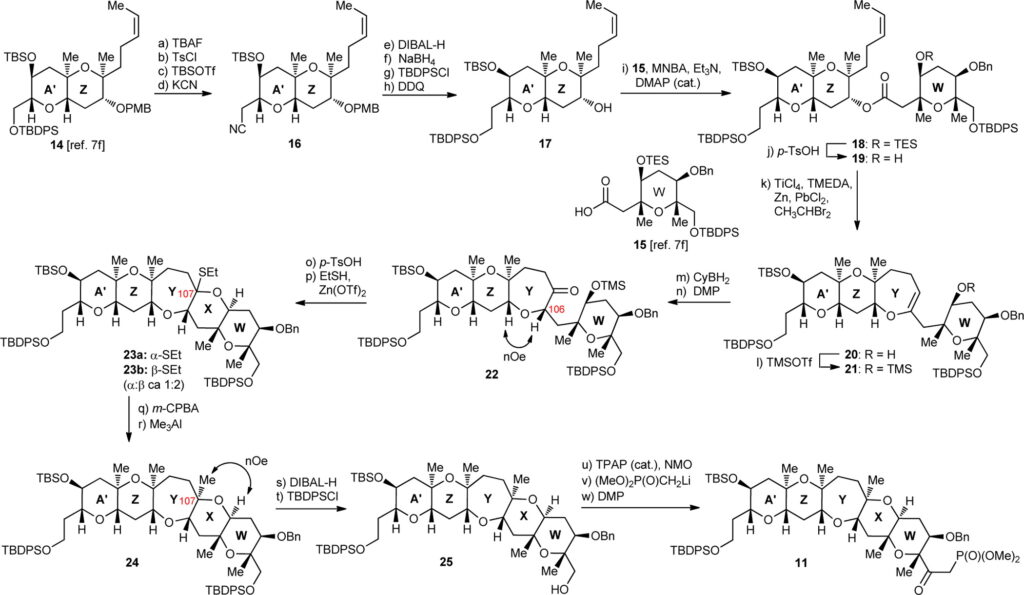
The synthetic strategy for the synthesis of discrete domains of maitotoxin, desired either for further elaboration into larger maitotoxin domains or to serve as tools for biological investigations, was devised based on the retrosynthetic analysis. Chemists began to play, initiating with ring dismantling, through several stereoselective hydroketone reductive cyclization, ultimately escorting the way to an enone moiety, as a potential precursor. Now, the ball is in the court of chemical substitutions and rearrangements, which witnessed the summarization of the ketophosphonate fragment construction, driven progressively through the art of monotosylation, resilylation, including stepwise reductions of the nitrile fragments by DIBAL-H and NaBH4, on a successive note. Steric hindrance and a plethora of other challenging aspects came to act in between, but still the final acetonide bis-benzyl ether domain was furnished with a triumphant ‘Eureka’ note!
The ‘Big Papas’ in Action:
There are many intracellular messengers present within our homeostatic physiological environment which help in mediating various responses. One such secondary intracellular messenger is Ca2+ ions that are responsible for carrying out diverse cellular responses like proliferation, contraction, secretion, fertilization and many more.
Maitotoxins have attracted huge attention from scientists as these molecules show powerful bioactivity involving the disruption of Ca2+ homeostasis. Thus, these toxin molecules serve as really important and versatile tools for studies of cellular events with intracellular Ca2+ changes like hormone secretion, apoptosis activation, etc.
From many earlier works, it became clear that Maitotoxins act on the mammalian cells, and on some solid pharmaceutical basis, it was proposed that these toxins activated the voltage-gated Ca2+ channels. Based on some of these reports on mechanisms of action of these toxins, some peculiar aspects of these toxins were also reported like delay in the induction of Ca2+ entry observed in many cell types, including inhibition of the Na+-K+-ATPase induced by the toxin.
Synthesis: A Roller Coaster Ride:
“In all chaos, there is a cosmos. In all disorder, a secret order.” ~ Carl Jung. This mess of gorgeous chaos is circumscribed within 32 fused rings including 32 ether cycles, 22 methyl groups, 28 hydroxyl groups and 2 sulphuric acid esters.
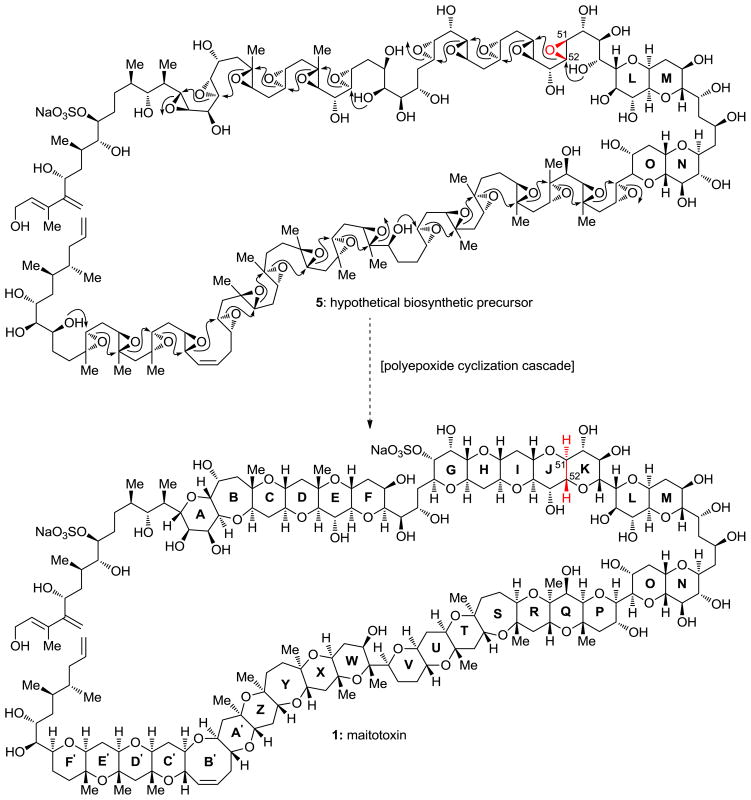
If we look at the biosynthesis of these ladder compounds, it was reported that these compounds are formed as a result of successive polyepoxide cascade ring openings. So this inspired the Nicolaou group to think of any possible biomimetic pathways to synthesize the molecule but unfortunately, it was impossible to implement any biomimetic synthetic methodology. As a consequence, the team planned to execute a convergent synthesis which indicates a conglomeration of different parts of the molecule separately, finally joining them to form the desired molecule at hand.
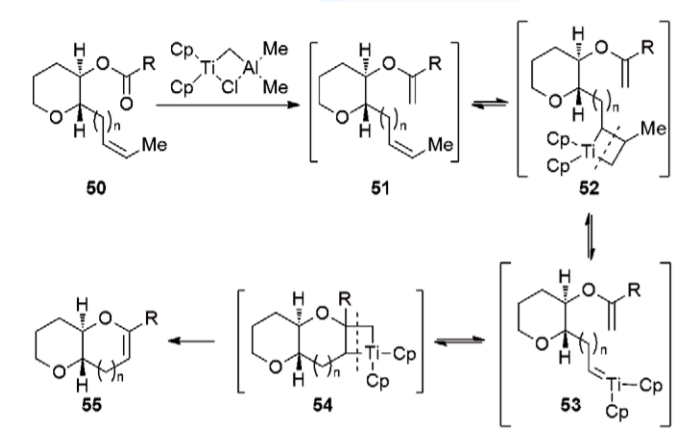
One of the most elementary and simple methodologies they developed was to use controlled ring closure reactions following the elementary Baldwin’s rules and it was found to be very effective for the formation of the cyclic ethers. They strategically induced an activating group adjacent to the far end of the epoxide group so that the 5-exo cyclisation gets blocked anyway and thus promoting the 6-endo cyclisation resulting in the formation of the required six-membered cyclic ether systems.
Another interesting reaction sequence came into action to join these cyclic ether skeletons. The ball was in the court of alkene metathesis reactions, namely the “Ester-Olefin ring closures”. The simplest way they used was to involve the Tebbe’s olefination reaction followed by subsequent thermal cyclizations and further doing preliminary hydroboration to get the extended ring framework.
But at the end, these synthetic methodologies teach us how simple reactions can be used to create such huge molecules with so much vivid and contrasting stereochemical features. How wonderful is the art of total synthesis!
Complexity: A ‘Nobel’ Tribute:
The art of simplicity is indeed a puzzle of complexity. Moreover, the beautiful ladder of this complex polyethereal architecture in the maitotoxin molecule appreciates its significance as a whole. The inspiration and lure to synthesize natural products was not more than that has been in that of maitotoxin molecule. The elegant molecular structure is indeed the representative of the most complex and diverse secondary metabolite in the world. Here, the highlights impact a blow on the importance of this molecule in spite of being a lethal poison and also, acts upon as an ode of humble tribute to the synthetic methods in Organic Chemistry which are continuously driving chemists to take their scribble pads and make out new pathways of synthesising new, more complex molecules. Building molecules is inevitably a difficult art. Dr. Benjamin List and Dr. David MacMillan are awarded the Nobel Prize in Chemistry 2021 for their development of a precise new tool for molecular construction: organocatalysis. An exciting and artistic journey indeed!
And don’t you dare to mess up with this freak! Or else, the result you know we guess!
Synthetically yours,
2 sulphur atoms!
REFERENCES:
- Encyclopaedia of Toxicology” – Philip Wexler, Bruce D. Anderson
- “Synthesis of Marine Polycyclic Polyethers via Endo-Selective Epoxide-Opening Cascades” – Ivan Vilotijevic, Timothy F. Jamison (2010)
- Synthesis and Biological Evaluation of QRSTUVWXYZA′ Domains of Maitotoxin” – K. C. Nicolaou et al. (2014)
- “Maitotoxin: An Inspiration for Synthesis” – K. C. Nicolaou, Robert J. Aversa
About the authors:

Sayantan is a 2nd Year BS-MS undergrad, a KVPY Fellow, and a budding Chem enthusiast, currently pursuing his studies in IISER Kolkata. Apart from being professionally passionate, he loves writing poems, reading storybooks, playing chess & cricket, or jamming with guitar in his leisure hours. He enjoys photography, blogging, the voyage of words on a pristine paper, the frolic of colours in his beaker, and exploring everything from quarks to quasars. He is an aspiring researcher in the field of chemical sciences and believes in the algorithm – ‘Do good, be good’. This is his third blog in The Qrius Rhino.

Subhajit is a 2nd-year BS-MS student at IISER Kolkata, an INSPIRE fellow, and an NSEC gold medalist. He is an enthusiastic science geek who loves
chemistry and aspires to do something in Natural Product Synthesis, Pericyclic chemistry, and Photochemistry. Apart from studies, being a music aficionado, and a trained Hindustani classical vocalist, he loves to sing and entertain his friend circle with lovely songs.